Insomnia medicine to decrease overactive wakefulness is approved
Idorsia Ltd announced that the European Commission (EC) has granted marketing authorisation for QUVIVIQ (daridorexant) for the treatment of adult patients with insomnia characterised by symptoms present for at least three months and considerable impact on daytime functioning.
Chronic insomnia disorder is one of the most prevalent sleep disorders in Europe, affecting between 6-12% of the adult population, and impacting both physical and mental health.

With this approval, QUVIVIQ becomes the first dual orexin receptor antagonist (DORA) in the European Union (EU) for the treatment of insomnia. Rather than inducing sleep through broad inhibition of brain activity, QUVIVIQ blocks only the activation of orexin receptors. Consequently, QUVIVIQ decreases the wake drive, allowing sleep to occur, without altering the proportion of sleep stages.
Jean-Paul Clozel, MD and chief executive officer of Idorsia, commented: “As our first treatment authorised in the EU, the approval of QUVIVIQ marks a significant medical advancement in the management of insomnia and a big milestone for Idorsia. We expect to make it available in the first countries before the end of the year.”
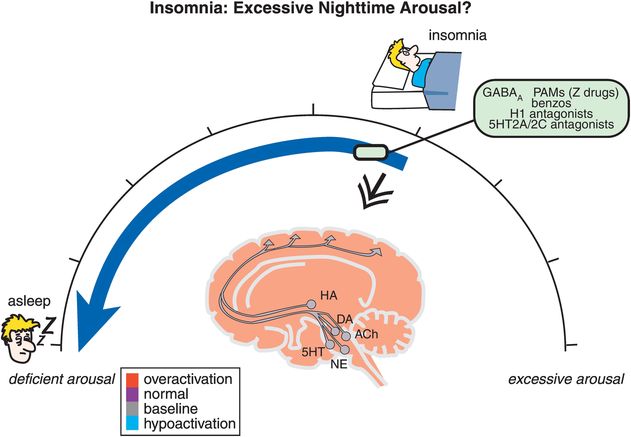
The EC decision is supported by robust Phase 3 results – published in The Lancet Neurology – which demonstrated that at the recommended dose, QUVIVIQ improved sleep onset, sleep maintenance and self-reported total sleep time in adults with chronic insomnia disorder. Patients reported feeling less mentally and physically tired, less sleepy and more energetic during the day, at months one and three compared to placebo, with a favourable safety profile. In clinical trials, the most frequently reported adverse reactions were headache and somnolence.
Professor Damien Léger, Université Paris Cité, France, commented: “QUVIVIQ, which can be used long-term, effectively improves sleep parameters and people’s ability to function better during the day, while avoiding major safety concerns, fulfilling the major medical requirements for insomnia management. This is great news for the millions of adults and elderly people across the EU living with chronic insomnia.”
Source: https://pharmaceuticalmanufacturer.media/
